TECVAYLI® step-up dosing1
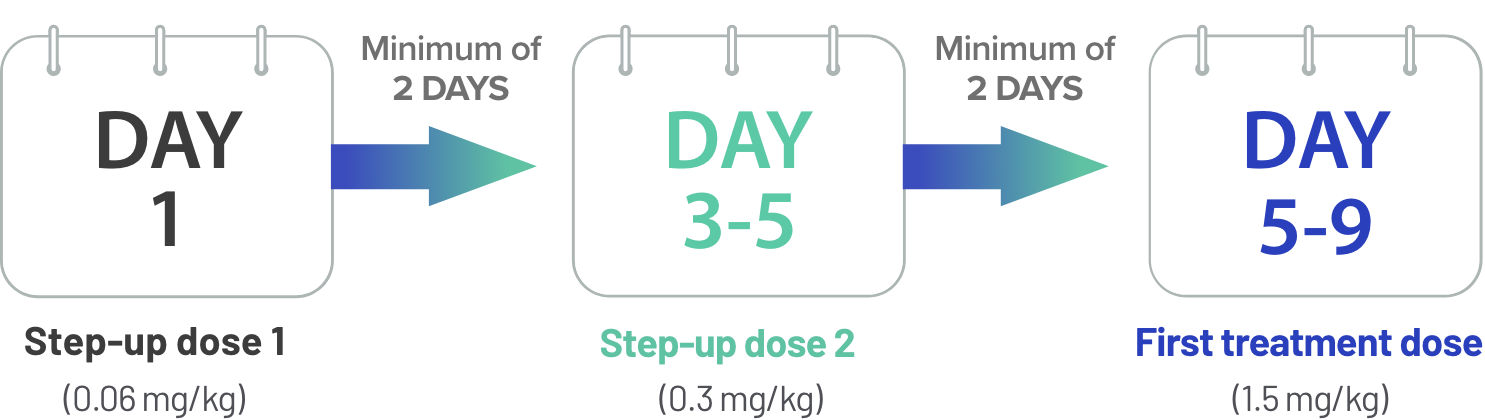

Step-up dose 2 may be given between 2 to 4 days after step-up dose 1 and may be given up to 7 days after step-up dose 1 to allow for resolution of adverse reactions
First treatment dose may be given between 2 to 4 days after step-up dose 2 and may be given up to 7 days after step-up dose 2 to allow for resolution of adverse reactions
Due to the risk of CRS and neurologic toxicity, including ICANS, patients should be hospitalized for 48 hours after administration of all doses within the TECVAYLI® step-up dosing schedule.
TECVAYLI® offers the ability to switch to a biweekly (Q2W) dosing option for certain patients1
After step-up dosing, patients will receive weekly treatment doses with the option of switching to biweekly (Q2W) dosing if they achieve and maintain ≥CR* for a minimum of 6 months.
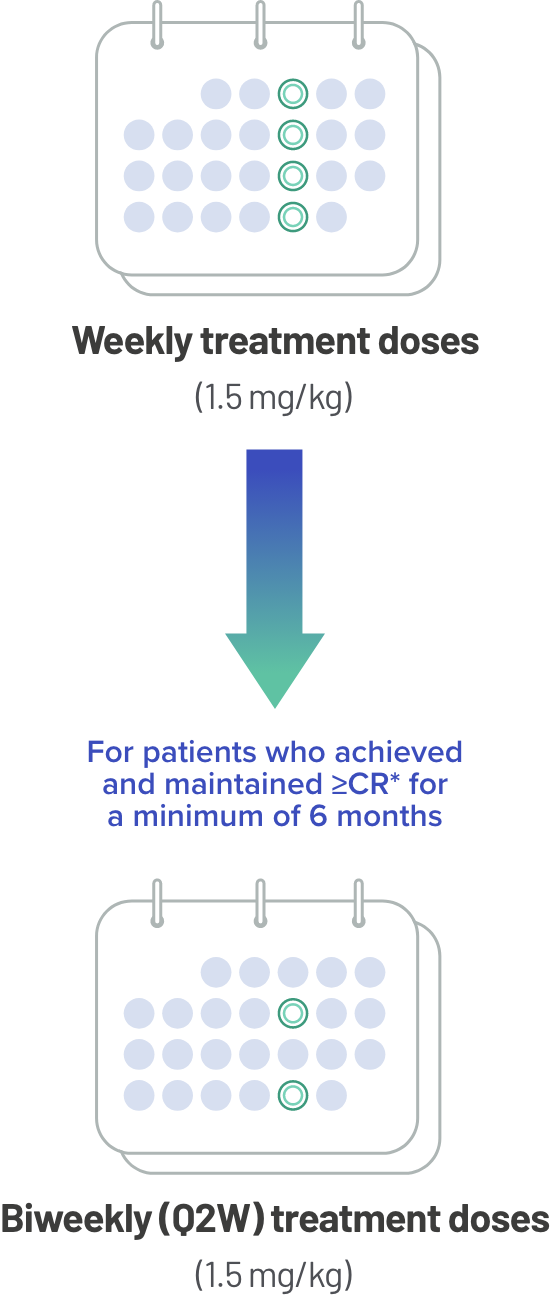

Weekly treatment doses(1.5 mg/kg)
Biweekly (Q2W) treatment doses(1.5 mg/kg)
Weekly Dosing: Once-weekly dosing until disease progression or unacceptable toxicity
Biweekly (Q2W) Dosing Option: Extended dosing interval beyond 6 months. The dosing frequency may be decreased to once every 2 weeks after ≥6 months of achieving and maintaining ≥CR* during treatment until disease progression or unacceptable toxicity
Remember: Dose is personalized to each patient’s actual body weight. Please refer to Tables 7-9 in the full Prescribing Information to determine the dosage based on predetermined weight ranges. Dose reductions are not recommended, and dose delays may be required to manage toxicities.
*≥CR: sCR+CR.
TECVAYLI® is administered by a healthcare provider according to the step-up dosing schedule to reduce incidence and severity of CRS.

Download the TECVAYLI® Dosing Guide for more information on weight-based dosing and administration
Pretreatment medications1
Prior to starting treatment with TECVAYLI®
Consider initiation of antiviral prophylaxis to prevent herpes zoster reactivation per local institutional guidelines.
1 to 3 hours before dose
Administer the following pretreatment medications of the TECVAYLI® step-up dosing schedule to reduce the risk of CRS.
- Corticosteroid (oral or intravenous dexamethasone 16 mg)
- Histamine-1 (H1) receptor antagonist (oral or intravenous diphenhydramine 50 mg or equivalent)
- Antipyretics (oral or intravenous acetaminophen 650 mg to 1000 mg or equivalent)
Prior to administration of weekly doses
Administration of pretreatment medications may be required prior to administration of subsequent doses of TECVAYLI® in the following patients:
- Patients who repeat doses within the step-up dosing schedule following a dose delay
- Patients who experienced CRS following the prior dose of TECVAYLI®
Dose Modifications for Adverse Reactions
Dosage reductions are not recommended with TECVAYLI®1
Dosage delays may be required to manage toxicities related to TECVAYLI®.
Dosage interruptions of TECVAYLI® due to adverse reactions occurred in 73% of patients in the primary analysis. Adverse reactions which required dosage interruption in >5% of patients included:
- Neutropenia
- Pneumonia
- Pyrexia
- CRS
- Upper respiratory tract infection
- COVID-19
1.2% of patients receiving TECVAYLI® in the primary analysis permanently discontinued treatment due to adverse reactions1
The adverse reactions resulting in permanent discontinuation of TECVAYLI® included pneumonia (adenoviral and pneumocystis jirovecii pneumonia in the same patient) and hypercalcemia.
5.5% of patients receiving TECVAYLI® in the final analysis permanently discontinued treatment due to adverse reactions2*
The adverse reactions resulting in permanent discontinuation of TECVAYLI® included COVID-19 in 2 patients; progressive multifocal leukoencephalopathy, sepsis, arthralgia, polyarthritis, and brain neoplasm in 1 patient each; events of pneumocystis jirovecii pneumonia and pneumonia adenoviral in the same patient; and hypercalcemia.
Dosage delays may be required to manage toxicities related to TECVAYLI®.1
*This information is not included in the current full Prescribing Information.


*Based on American Society for Transplantation and Cellular Therapy (ASTCT) 2019 grading for CRS.
†Attributed to CRS. Fever may not always be present concurrently with hypotension or hypoxia as it may be masked by interventions such as antipyretics or anticytokine therapy.
‡See Table 2 of the full Prescribing Information for recommendations on restarting TECVAYLI® after dose delays.
§Low-flow nasal cannula is ≤6 L/min, and high-flow nasal cannula is >6 L/min.


*Based on National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03.
†See Table 2 of the full Prescribing Information for recommendations on restarting TECVAYLI® after dose delays.
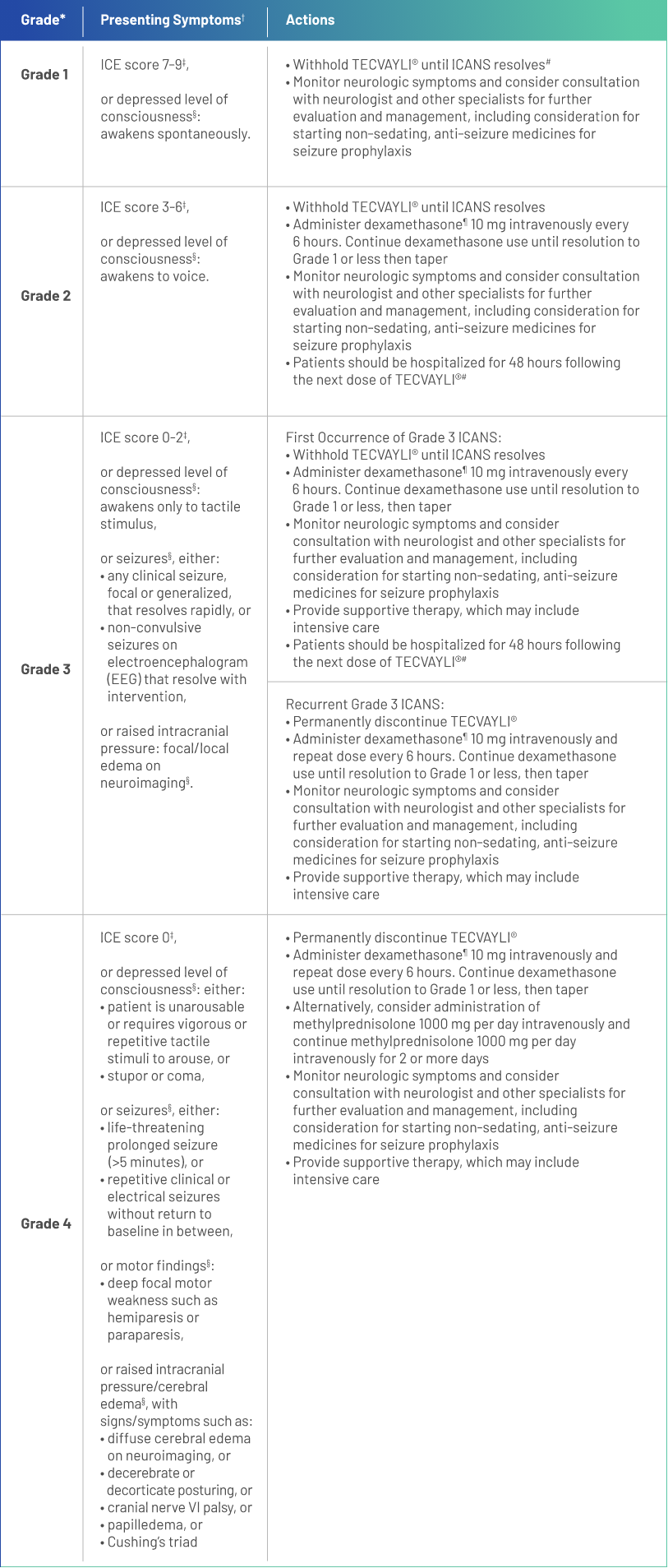
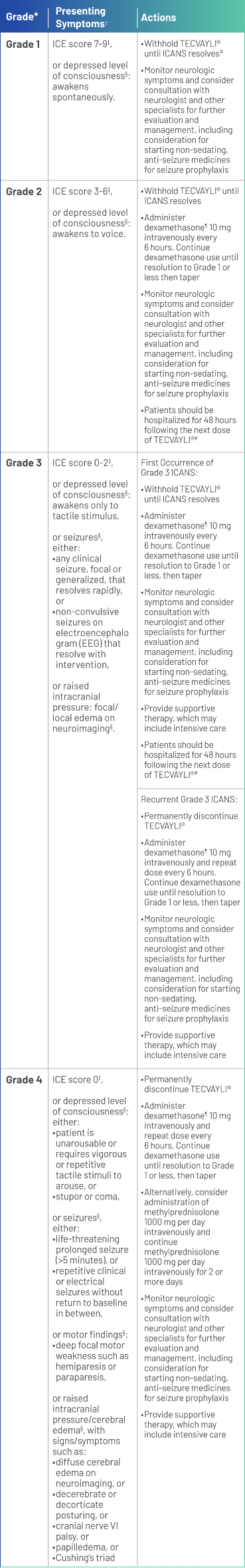
*Based on American Society for Transplantation and Cellular Therapy (ASTCT) 2019 grading for ICANS.
†Management is determined by the most severe event, not attributable to any other cause.
‡If patient is arousable and able to perform Immune Effector Cell-Associated Encephalopathy (ICE) Assessment, assess: Orientation (oriented to year, month, city, hospital = 4 points); Naming (name 3 objects, eg, point to clock, pen, button = 3 points); Following Commands (eg, “show me 2 fingers” or “close your eyes and stick out your tongue” = 1 point); Writing (ability to write a standard sentence = 1 point; and Attention (count backwards from 100 by ten = 1 point). If patient is unarousable and unable to perform ICE Assessment (Grade 4 ICANS) = 0 points.
§Not attributable to any other cause.
¶All references to dexamethasone administration are dexamethasone or equivalent.
#See Table 2 of the full Prescribing Information for recommendations on restarting TECVAYLI® after dose delays.
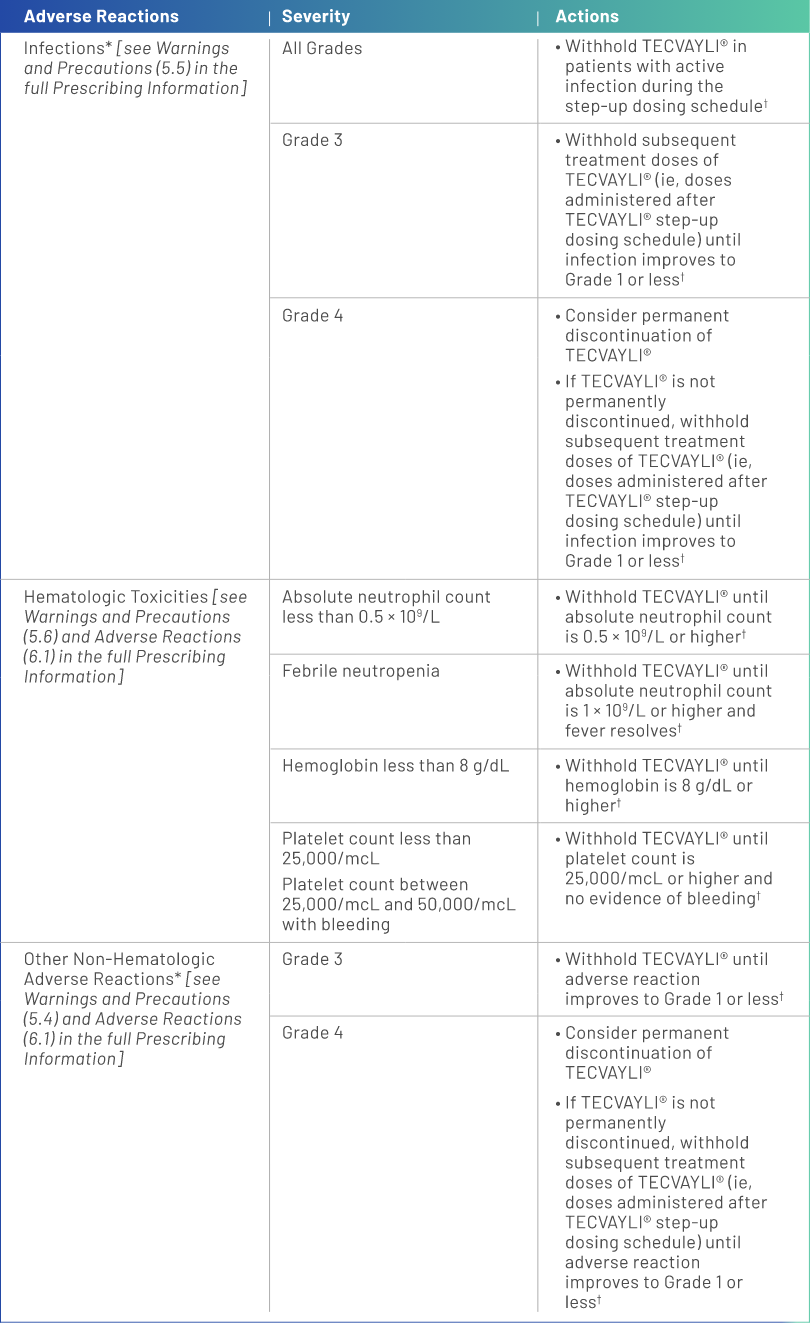

*Based on National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03.
†See Table 2 of the full Prescribing Information for recommendations on restarting TECVAYLI® after dose delays.
BCMA, B-cell maturation antigen; COVID-19, coronavirus disease 2019; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ICE, immune effector cell-associated encephalopathy.
References:
- TECVAYLI® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Data on file. Janssen Biotech, Inc.