The binding of TECVAYLI® to both BCMA and CD3 leads to the activation of T-cells, resulting in the lysis of multiple myeloma cells
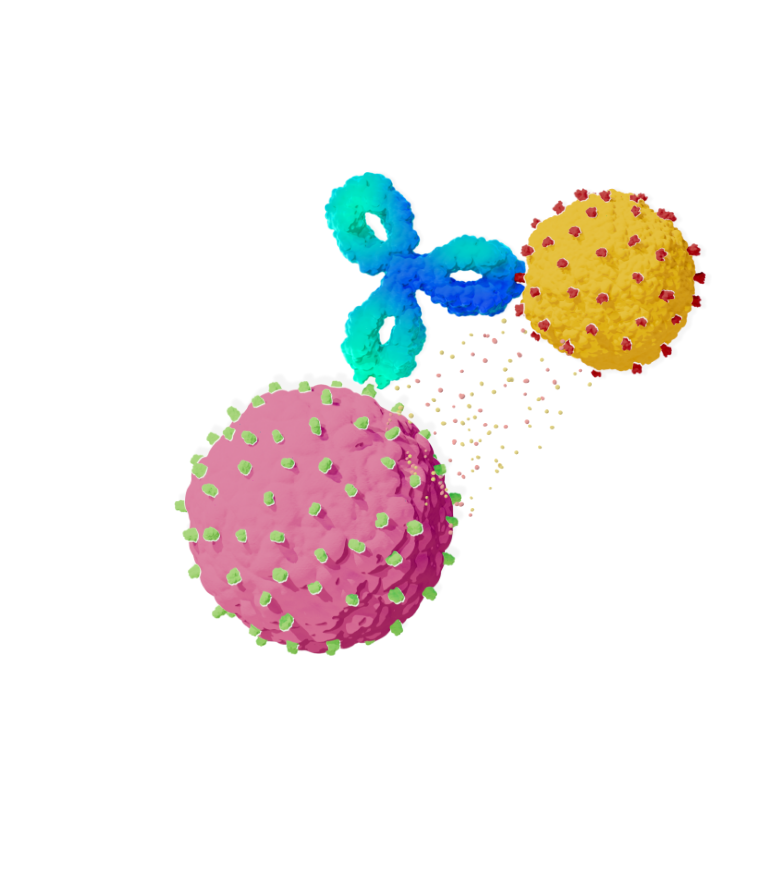
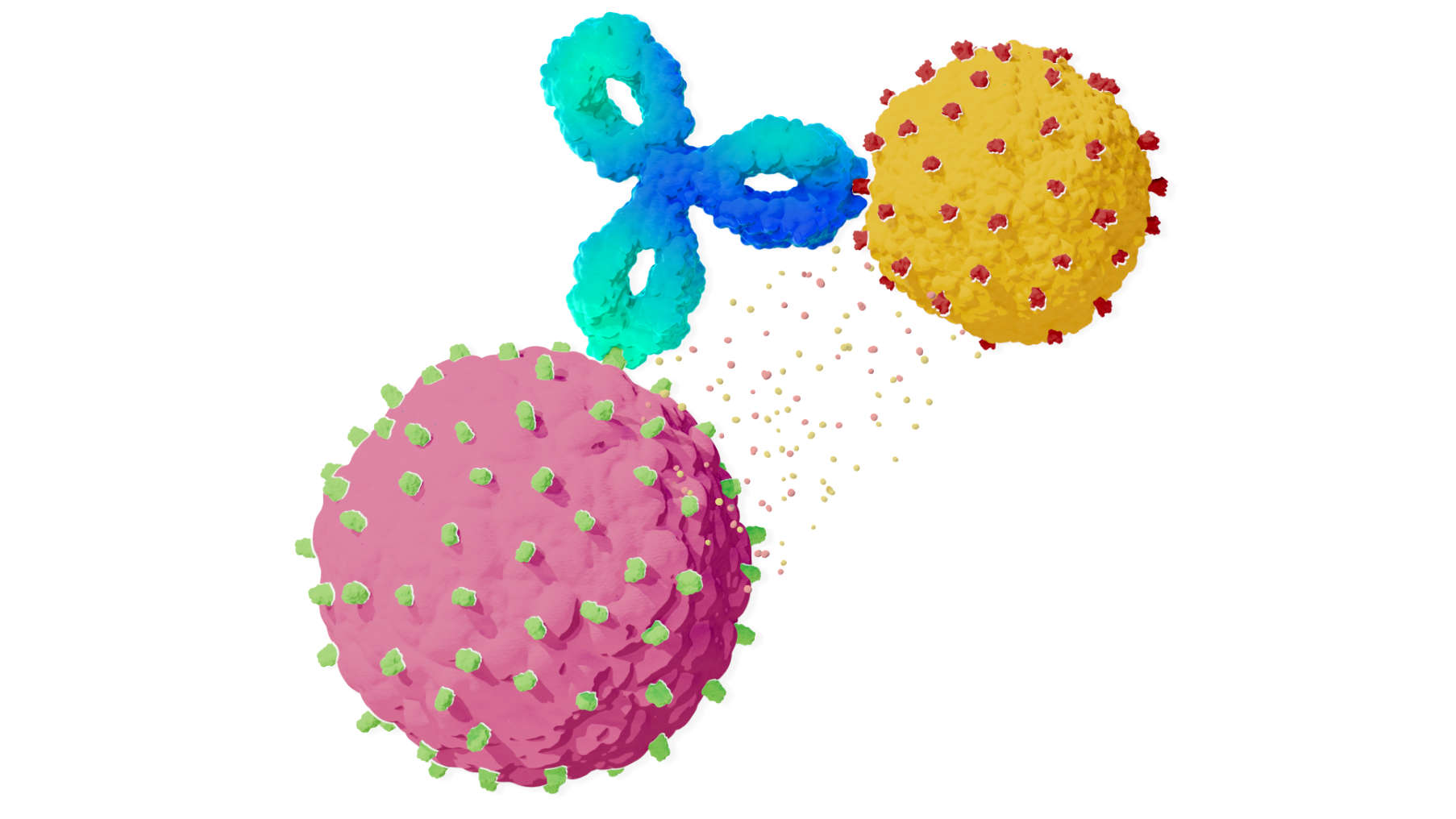
TECVAYLI®
CD3
Immune Cell
BCMA
Cancer Cell
The CD3-targeting arm of TECVAYLI® binds to CD3 on the surface of the T-cell
The BCMA-targeting arm of TECVAYLI® binds to BCMA
on the surface of MM cells and some healthy
B-lineage cells
Lysis of multiple myeloma cells results from the activation of T-cells and the release of various proinflammatory cytokines
BCMA, B-cell maturation antigen; CD3, cluster of differentiation 3; MM, multiple myeloma.
References:
- TECVAYLI® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- U.S. Food and Drug Administration. FDA approves teclistamab-cqyv for relapsed or refractory multiple myeloma. Accessed June 24, 2025. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-teclistamab-cqyv-relapsed-or-refractory-multiple-myeloma